The importance of efficiency in water electrolysis ⚡💧
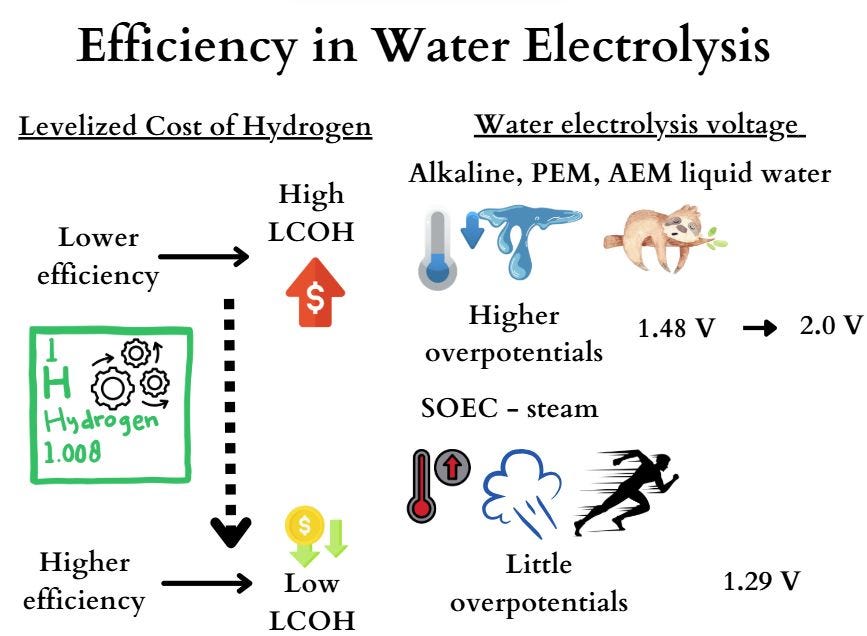
One of the main nightmares for hydrogen developers is the high Levelized Cost of Hydrogen (LCOH). As I have discussed in many of my previous posts, OPEX is the key driver behind LCOH — and the most effective way to reduce it is by improving efficiency.
But why is efficiency so critical? The answer lies in electrochemistry.
🔋 For water electrolysis with liquid electrolytes (Alkaline, PEM, AEM), the process operates at relatively low temperatures. This results in higher overpotentials, pushing the required cell voltage from the theoretical 1.48 V up to around 2.0 V. That gap represents energy losses — and higher electricity bills.
🔥 In contrast, SOEC (Solid Oxide Electrolysis Cells) uses steam at high temperatures. Here, the system faces much lower overpotentials, with operating voltages closer to 1.29 V. This means less wasted energy and significantly higher efficiency.
👉 The bottom line: higher efficiency = lower LCOH.
For hydrogen to scale competitively, improving electrolysis efficiency is not just a technical challenge — it is an economic necessity.
What do you think — will efficiency gains in electrolysis be enough to bring LCOH to competitive levels, or will we need stronger policy and market interventions?
P.S. The text of the post has been generated 100 % by AI with hopefully a nice prompt, 🙃 do you like it or you prefer other style?
P.S. 2. The image is 100 % genuine and made by me using Canva. 😊