The following article has been written by Ager Prieto Elorduy, author of the newsletter “Energy Analysis”.
Ager works as a process engineer on hydrogen issues at the Spanish Engineering Sener, and has recently completed the pioneering inter-university master's degree at the Spanish level on hydrogen technologies at the University of Mondragón.
Enjoy!
Today, I am going to talk about what EFFECT HYDROGEN BLENDING HAS ON NATURAL GAS, analyzing how the most significant properties change for its use as fuel in industry or residential use.
Natural gas consists of a mixture of gases, in variable proportions, but where methane (CH₄) constitutes the main element with a percentage greater than 90%, and the rest other gaseous hydrocarbons such as ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀) and/or nitrogen (N₂) or hydrogen (H₂), among others.
For the case study that I am going to describe in the following lines, I have established a 100% methane stream, this being a correct assumption, given that I have verified the calculations with real compositions of natural gas, and the results are the same.
Similarly, I want to highlight that the properties of the gas are linked to the temperature and pressure at which the gas mixture is found. That is why I am going to highlight that the calculations that I present here have been carried out at 15ºC (288 K) and 1 bar (0.1 MPa).
Context of Blending
Obviously, in a decarbonized world, the large gas infrastructure that Spain has is condemned to irrelevance if fossil fuels are discarded. In this scenario, the addition of hydrogen to natural gas is being considered to take advantage of the pipelines that are in operation. The combustion of hydrogen (H₂), since it does not have carbon in its structure, does not emit carbon dioxide (CO₂). Thus, there are many who advocate hydrogen blending to decarbonize distribution networks, and its subsequent downstream uses.
Through the analysis that you are going to read below, I want you all to have OBJECTIVE DATA WITH TECHNICAL RIGOR so that you know the real implications of blending in natural gas.
When I started doing the calculations, unconsciously, my head expected to end up with a graph with this linear trend. Where, every 10% by volume of hydrogen that we include in natural gas would reduce CO₂ emissions by 10% by volume.
Figure 1. Trend of CO₂ reduction with hydrogen blending. (Source: self made).
But my conclusions were not entirely correct. Now you will understand why. The graph itself is fine, since, if I remove 10% by volume of CH₄ in the initial mixture, to add another 10% by volume of H₂ (which DOES NOT emit CO₂), obviously, CO₂ emissions will be reduced by a 10%. So far, everyone agrees, right?
But because I say that the conclusions were not entirely correct, because in that graph I have not taken into consideration the changes that occur in the calorific value in the new mixture that is generated. Therefore, in the graph described above, the emissions generated decrease linearly, but the gas mixture generated by the hydrogen bleed does NOT provide the same heat as the initial stream of 100% CH₄. We must not forget that the purpose of combustion is always the same, to obtain heat, whether for an industrial process or for heating; but above all, a constant heat.
To understand this, it is best to analyze the differences between the two gases in their pure states, as you can see in the following table, due to their nature, they have different characteristics.
Table 1. Intrinsic properties of methane and hydrogen. (Source: self made).
In the following graph you can see how the molecular weight of the H₂-CH₄ mixture changes, and similarly, the evolution of the mass heating value of the mixture.
Figure 3. Variation of the hydrogen/methane mixture and its impact on the molecular weight and mass calorific value. (Source: self made).
The molecular weight of the mixture drops steadily, as we gradually include hydrogen into the mixture, pure arithmetic. On the other hand, the mass heating value is greatly influenced by the mass energy density of hydrogen. Let us not forget that hydrogen weighs very little and occupies a lot of volume under atmospheric conditions.
What happens to the mass flow when hydrogen blending?
Therefore, I have established a 1 MW boiler that provides us with heat (1000 MJ/h) as the objective of the mixture, in order to normalize the data taking into account the variation of each of the components. In this way, with the initial flow of 100% CH₄, we will need a mass flow rate of 17.994 kg/h to obtain the set heat of 1000 MJ/h in the burner.
What is the first peculiarity that crosses our path, as we include 1, 2, 3, 4, 5... 99% of Hydrogen, and reducing the CH₄ content in equal proportion, (for example 99% CH₄ + 1 % H₂, 98% CH₄ + 2% H₂ … ), that the molecular weight of hydrogen is 8 times less than methane. Therefore, when we look at the mass flow rate (that is, the mass flow rate), adding hydrogen has little impact, as you can see in the following graph.
Figure 4. Behavior of the mass flow when hydrogen blending to achieve the same heat of combustion. (Source: self made).
As you can see, from the initial stream of 100% CH₄ to the final stream of 100% H₂, 2.55 times LESS mass is needed to obtain the same heat in combustion. However, hydrogen is not in a solid state, but in a gas. Therefore, it would be more appropriate to speak in volume rather than in mass.
But, Ager, then what happens to the molar flow rate when blending hydrogen?
Well, in that case it's different. Since, in the mass flow, the gravimetric energy density (MJ/kg) was relevant, but in the molar flow, what is relevant is the volumetric energy density (MJ/m³). In this way, with the initial flow of 100% CH₄, we will need a mass flow rate of 1.122 kmol/h to obtain the set heat of 1000 MJ/h in combustion.
You will all remember that at school in the Chemistry subject the law of ideal gases was taught, which was governed by PV = n RT. From this formula we can conclude that 1 mole of gas occupies 22.4 L at standard conditions of pressure and temperature.
Figure 5, Behavior of the molar flow rate when hydrogen is blended to achieve the same heat of combustion. (Source: self made).
As you can see, from the initial stream of 100% CH₄ to the final stream of 100% H₂, 3.11 times MORE moles (= volume) are needed to obtain the same heat in combustion.
So, what we observed, that to achieve the same heat that we achieved with 100% CH₄, the trend of reducing CO₂ emissions when blending Hydrogen, IS NOT LINEAR, it has THE SHAPE OF A PARABOLIC SHOT.
6.Image.- Reduction of CO₂ emissions when blending hydrogen. (Source: self made).
Decarbonization achieved by hydrogen blending
In the following graph we can see how little hydrogen blending decarbonizes in a 100% methane stream. Consider that natural gas is +90% methane, and the rest is made up of other hydrocarbons such as ethane, propane, butane... So, we are facing the same scenario.
Figure 7. Decarbonization achieved by hydrogen blending. (Source: self made).
As you can see in the graph:
-A 25% H₂ addiction decarbonizes 10%.
-A 50% H₂ addiction decarbonizes 24%.
-75% H₂ addiction decarbonizes 48%.
Therefore, if we want to make natural gas (fossil fuel) more sustainable, hydrogen blending has a limited impact. Since, as has been demonstrated during this analysis, the emissions reduction achieved is minimal. Another different thing is that it is a 100% hydrogen current, that does decarbonize.
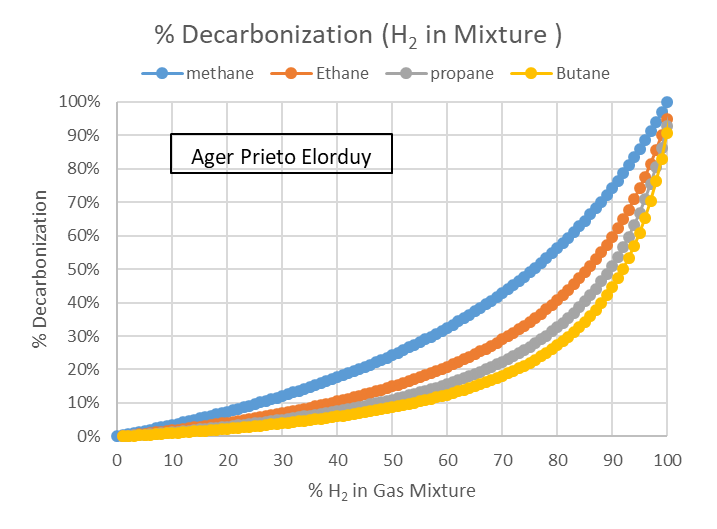
To conclude, and in case anyone is curious like me, this is the decarbonization generated by the blending of hydrogen in the rest of the gaseous hydrocarbons (100% currents).
Figure 8. Decarbonization achieved with the blending of hydrogen in different pure hydrocarbon streams. (Source: self made)
It is evident that the higher the molecular weight of the gaseous hydrocarbon, the lower the decarbonization that is achieved with hydrogen blending, and therefore, the lower the reduction in CO₂ emissions associated with it.












Great article. Maybe the below article can be also useful
https://seanalytics.substack.com/p/hydrogen-utilization-in-gas-turbine